adaptivebiotech.com/pipeline
Preview meta tags from the adaptivebiotech.com website.
Linked Hostnames
10- 33 links towww.adaptivebiotech.com
- 5 links toadaptivebiotech.com
- 4 links toinvestors.adaptivebiotech.com
- 1 link toclonoseq.com
- 1 link totwitter.com
- 1 link towww.clonoseq.com
- 1 link towww.facebook.com
- 1 link towww.instagram.com
Thumbnail
Search Engine Appearance
MRD Pipeline
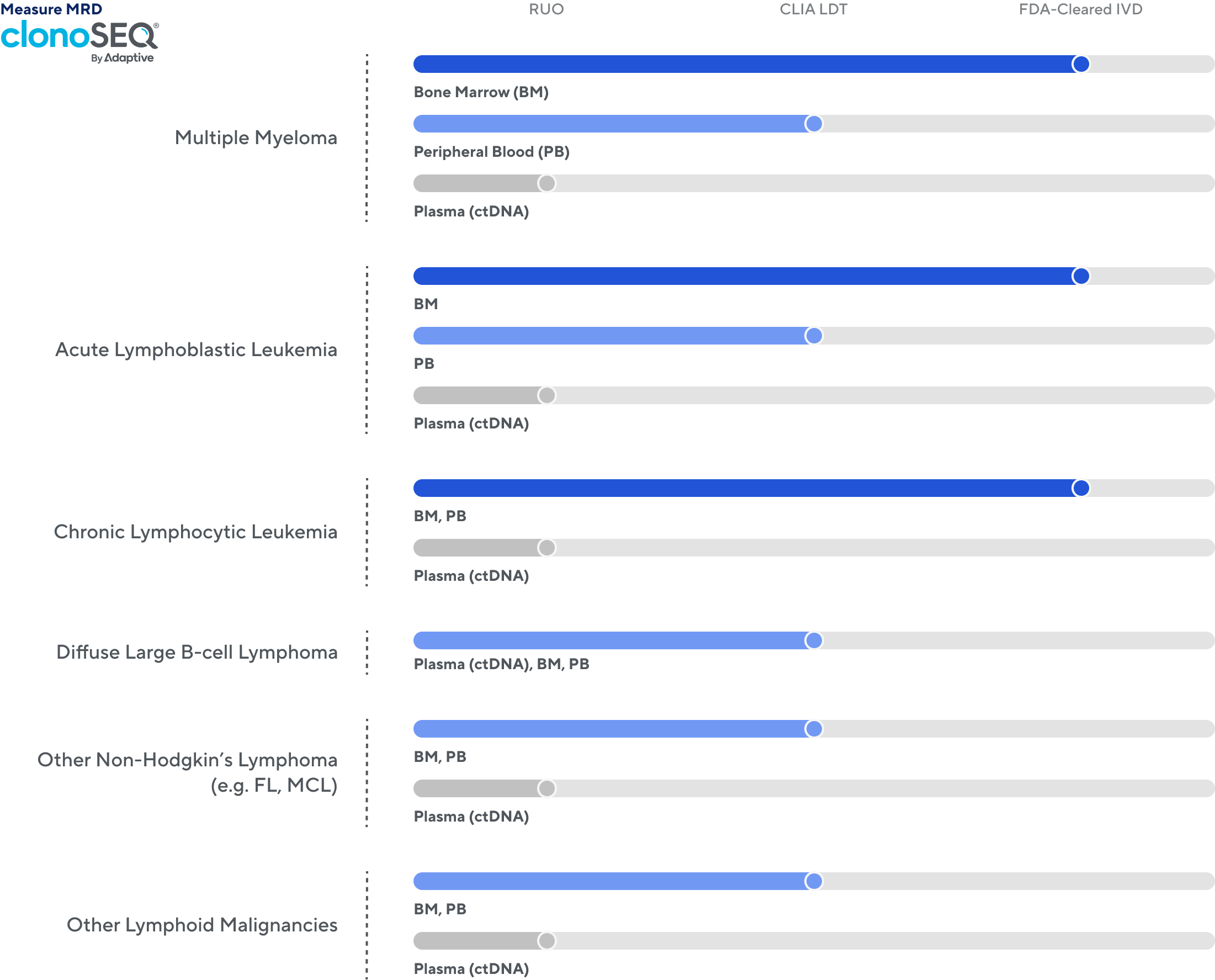
1 Available to order as a CLIA-validated laboratory developed test (LDT) service. This use has not been cleared or approved by the FDA. clonoSEQ® is available as an FDA-cleared in vitro diagnostic (IVD) test service provided by Adaptive Biotechnologies to detect minimal residual disease (MRD) in bone marrow from patients with multiple myeloma or B-cell acute lymphoblastic leukemia […]
Bing
MRD Pipeline
1 Available to order as a CLIA-validated laboratory developed test (LDT) service. This use has not been cleared or approved by the FDA. clonoSEQ® is available as an FDA-cleared in vitro diagnostic (IVD) test service provided by Adaptive Biotechnologies to detect minimal residual disease (MRD) in bone marrow from patients with multiple myeloma or B-cell acute lymphoblastic leukemia […]
DuckDuckGo
MRD Pipeline
1 Available to order as a CLIA-validated laboratory developed test (LDT) service. This use has not been cleared or approved by the FDA. clonoSEQ® is available as an FDA-cleared in vitro diagnostic (IVD) test service provided by Adaptive Biotechnologies to detect minimal residual disease (MRD) in bone marrow from patients with multiple myeloma or B-cell acute lymphoblastic leukemia […]
General Meta Tags
8- titleMRD Pipeline - Adaptive Biotech
- charsetUTF-8
- viewportwidth=device-width, initial-scale=1
- robotsindex, follow, max-image-preview:large, max-snippet:-1, max-video-preview:-1
- article:publisherhttps://www.facebook.com/adaptivebiotech/
Open Graph Meta Tags
10og:locale
en_US- og:typearticle
- og:titleMRD Pipeline
- og:description1 Available to order as a CLIA-validated laboratory developed test (LDT) service. This use has not been cleared or approved by the FDA. clonoSEQ® is available as an FDA-cleared in vitro diagnostic (IVD) test service provided by Adaptive Biotechnologies to detect minimal residual disease (MRD) in bone marrow from patients with multiple myeloma or B-cell acute lymphoblastic leukemia […]
- og:urlhttps://www.adaptivebiotech.com/mrd-pipeline/
Twitter Meta Tags
2- twitter:cardsummary_large_image
- twitter:site@AdaptiveBiotech
Link Tags
24- EditURIhttps://www.adaptivebiotech.com/xmlrpc.php?rsd
- alternatehttps://www.adaptivebiotech.com/feed/
- alternatehttps://www.adaptivebiotech.com/comments/feed/
- alternatehttps://www.adaptivebiotech.com/wp-json/wp/v2/pages/26804
- alternatehttps://www.adaptivebiotech.com/wp-json/oembed/1.0/embed?url=https%3A%2F%2Fwww.adaptivebiotech.com%2Fmrd-pipeline%2F
Links
49- http://investors.adaptivebiotech.com
- https://adaptivebiotech.com/pipeline#diagnostics
- https://adaptivebiotech.com/pipeline/#diagnostics
- https://adaptivebiotech.com/publications
- https://adaptivebiotech.com/publications/?_sft_publication_categories=immune-medicine