www.nature.com/articles/s41586-025-08978-0
Preview meta tags from the www.nature.com website.
Linked Hostnames
32- 173 links towww.nature.com
- 52 links todoi.org
- 51 links toscholar.google.com
- 32 links towww.ncbi.nlm.nih.gov
- 24 links toadsabs.harvard.edu
- 12 links toscholar.google.co.uk
- 7 links toorcid.org
- 7 links tostatic-content.springer.com
Thumbnail
Search Engine Appearance
Encapsulated Co–Ni alloy boosts high-temperature CO2 electroreduction - Nature
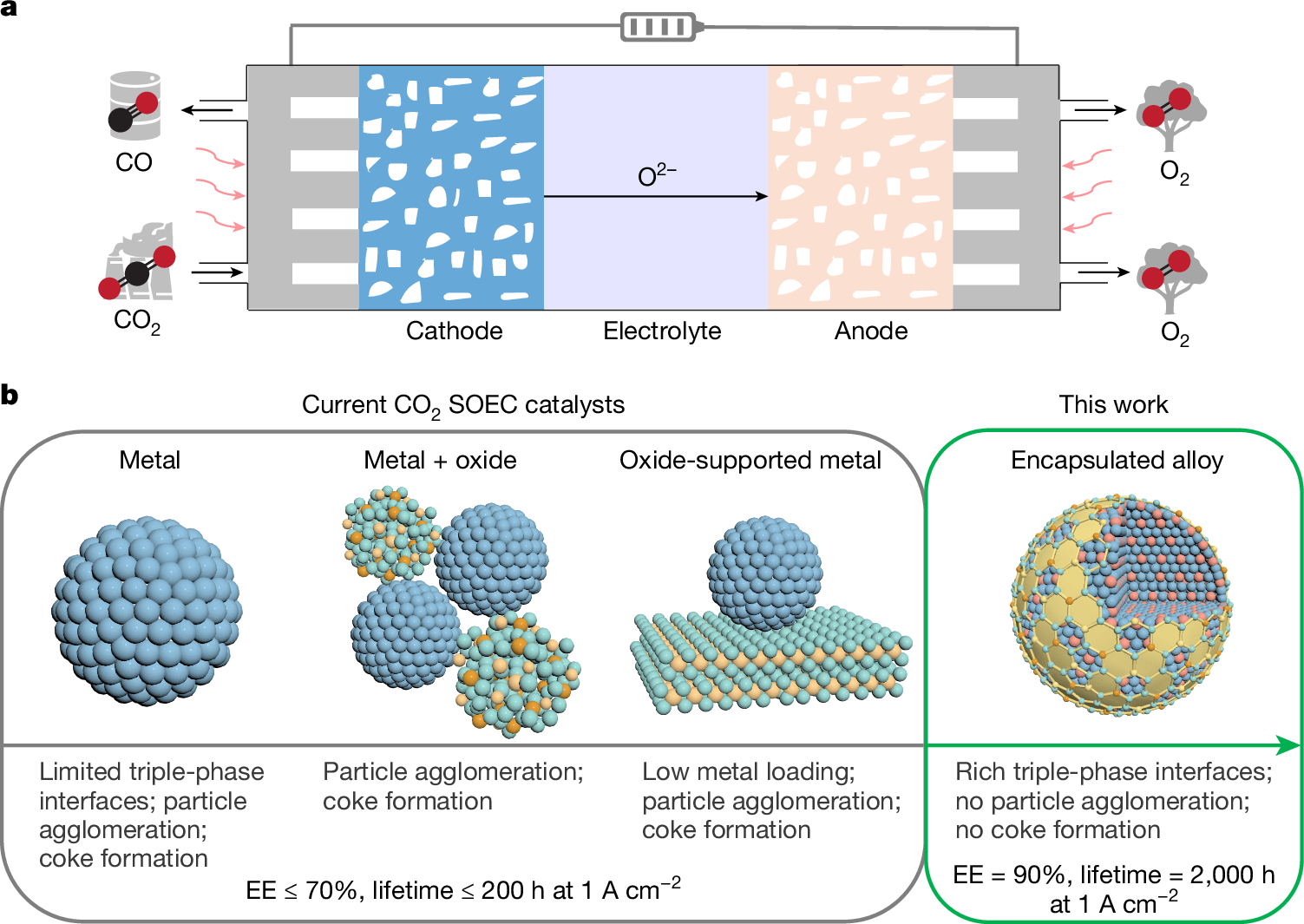
Electrochemical CO2 reduction into chemicals and fuels holds great promise for renewable energy storage and carbon recycling1–3. Although high-temperature CO2 electroreduction in solid oxide electrolysis cells is industrially relevant, current catalysts have modest energy efficiency and a limited lifetime at high current densities, generally below 70% and 200 h, respectively, at 1 A cm−2 and temperatures of 800 °C or higher4–8. Here we develop an encapsulated Co–Ni alloy catalyst using Sm2O3-doped CeO2 that exhibits an energy efficiency of 90% and a lifetime of more than 2,000 h at 1 A cm−2 for high-temperature CO2-to-CO conversion at 800 °C. Its selectivity towards CO is about 100%, and its single-pass yield reaches 90%. We show that the efficacy of our catalyst arises from its unique encapsulated structure and optimized alloy composition, which simultaneously enable enhanced CO2 adsorption, moderate CO adsorption and suppressed metal agglomeration. This work provides an efficient strategy for the design of catalysts for high-temperature reactions that overcomes the typical trade-off between activity and stability and has potential industrial applications. An optimized Co–Ni alloy catalyst encapsulated with Sm2O3-doped CeO2 shows both high activity and stability for high-temperature CO2-to-CO conversion, overcoming the limitations of such catalysts typically used in industrial applications.
Bing
Encapsulated Co–Ni alloy boosts high-temperature CO2 electroreduction - Nature
Electrochemical CO2 reduction into chemicals and fuels holds great promise for renewable energy storage and carbon recycling1–3. Although high-temperature CO2 electroreduction in solid oxide electrolysis cells is industrially relevant, current catalysts have modest energy efficiency and a limited lifetime at high current densities, generally below 70% and 200 h, respectively, at 1 A cm−2 and temperatures of 800 °C or higher4–8. Here we develop an encapsulated Co–Ni alloy catalyst using Sm2O3-doped CeO2 that exhibits an energy efficiency of 90% and a lifetime of more than 2,000 h at 1 A cm−2 for high-temperature CO2-to-CO conversion at 800 °C. Its selectivity towards CO is about 100%, and its single-pass yield reaches 90%. We show that the efficacy of our catalyst arises from its unique encapsulated structure and optimized alloy composition, which simultaneously enable enhanced CO2 adsorption, moderate CO adsorption and suppressed metal agglomeration. This work provides an efficient strategy for the design of catalysts for high-temperature reactions that overcomes the typical trade-off between activity and stability and has potential industrial applications. An optimized Co–Ni alloy catalyst encapsulated with Sm2O3-doped CeO2 shows both high activity and stability for high-temperature CO2-to-CO conversion, overcoming the limitations of such catalysts typically used in industrial applications.
DuckDuckGo
Encapsulated Co–Ni alloy boosts high-temperature CO2 electroreduction - Nature
Electrochemical CO2 reduction into chemicals and fuels holds great promise for renewable energy storage and carbon recycling1–3. Although high-temperature CO2 electroreduction in solid oxide electrolysis cells is industrially relevant, current catalysts have modest energy efficiency and a limited lifetime at high current densities, generally below 70% and 200 h, respectively, at 1 A cm−2 and temperatures of 800 °C or higher4–8. Here we develop an encapsulated Co–Ni alloy catalyst using Sm2O3-doped CeO2 that exhibits an energy efficiency of 90% and a lifetime of more than 2,000 h at 1 A cm−2 for high-temperature CO2-to-CO conversion at 800 °C. Its selectivity towards CO is about 100%, and its single-pass yield reaches 90%. We show that the efficacy of our catalyst arises from its unique encapsulated structure and optimized alloy composition, which simultaneously enable enhanced CO2 adsorption, moderate CO adsorption and suppressed metal agglomeration. This work provides an efficient strategy for the design of catalysts for high-temperature reactions that overcomes the typical trade-off between activity and stability and has potential industrial applications. An optimized Co–Ni alloy catalyst encapsulated with Sm2O3-doped CeO2 shows both high activity and stability for high-temperature CO2-to-CO conversion, overcoming the limitations of such catalysts typically used in industrial applications.
General Meta Tags
148- titleEncapsulated Co–Ni alloy boosts high-temperature CO2 electroreduction | Nature
- titleClose banner
- titleClose banner
- X-UA-CompatibleIE=edge
- applicable-devicepc,mobile
Open Graph Meta Tags
6- og:urlhttps://www.nature.com/articles/s41586-025-08978-0
- og:typearticle
- og:site_nameNature
- og:titleEncapsulated Co–Ni alloy boosts high-temperature CO2 electroreduction - Nature
- og:descriptionAn optimized Co–Ni alloy catalyst encapsulated with Sm2O3-doped CeO2 shows both high activity and stability for high-temperature CO2-to-CO conversion, overcoming the limitations of such catalysts typically used in industrial applications.
Twitter Meta Tags
6- twitter:site@nature
- twitter:cardsummary_large_image
- twitter:image:altContent cover image
- twitter:titleEncapsulated Co–Ni alloy boosts high-temperature CO2 electroreduction
- twitter:descriptionNature - An optimized Co–Ni alloy catalyst encapsulated with Sm2O3-doped CeO2 shows both high activity and stability for high-temperature CO2-to-CO conversion, overcoming the limitations of...
Item Prop Meta Tags
4- position1
- position2
- position3
- publisherSpringer Nature
Link Tags
15- alternatehttps://www.nature.com/nature.rss
- apple-touch-icon/static/images/favicons/nature/apple-touch-icon-f39cb19454.png
- canonicalhttps://www.nature.com/articles/s41586-025-08978-0
- icon/static/images/favicons/nature/favicon-48x48-b52890008c.png
- icon/static/images/favicons/nature/favicon-32x32-3fe59ece92.png
Emails
1Links
394- http://adsabs.harvard.edu/cgi-bin/nph-data_query?link_type=ABSTRACT&bibcode=1964RSPSA.277..237H
- http://adsabs.harvard.edu/cgi-bin/nph-data_query?link_type=ABSTRACT&bibcode=1976PhRvB..13.5188M
- http://adsabs.harvard.edu/cgi-bin/nph-data_query?link_type=ABSTRACT&bibcode=1994PhRvB..5017953B
- http://adsabs.harvard.edu/cgi-bin/nph-data_query?link_type=ABSTRACT&bibcode=1995PhRvB..51.4014M
- http://adsabs.harvard.edu/cgi-bin/nph-data_query?link_type=ABSTRACT&bibcode=1996PhRvB..5411169K